Certification of Factory
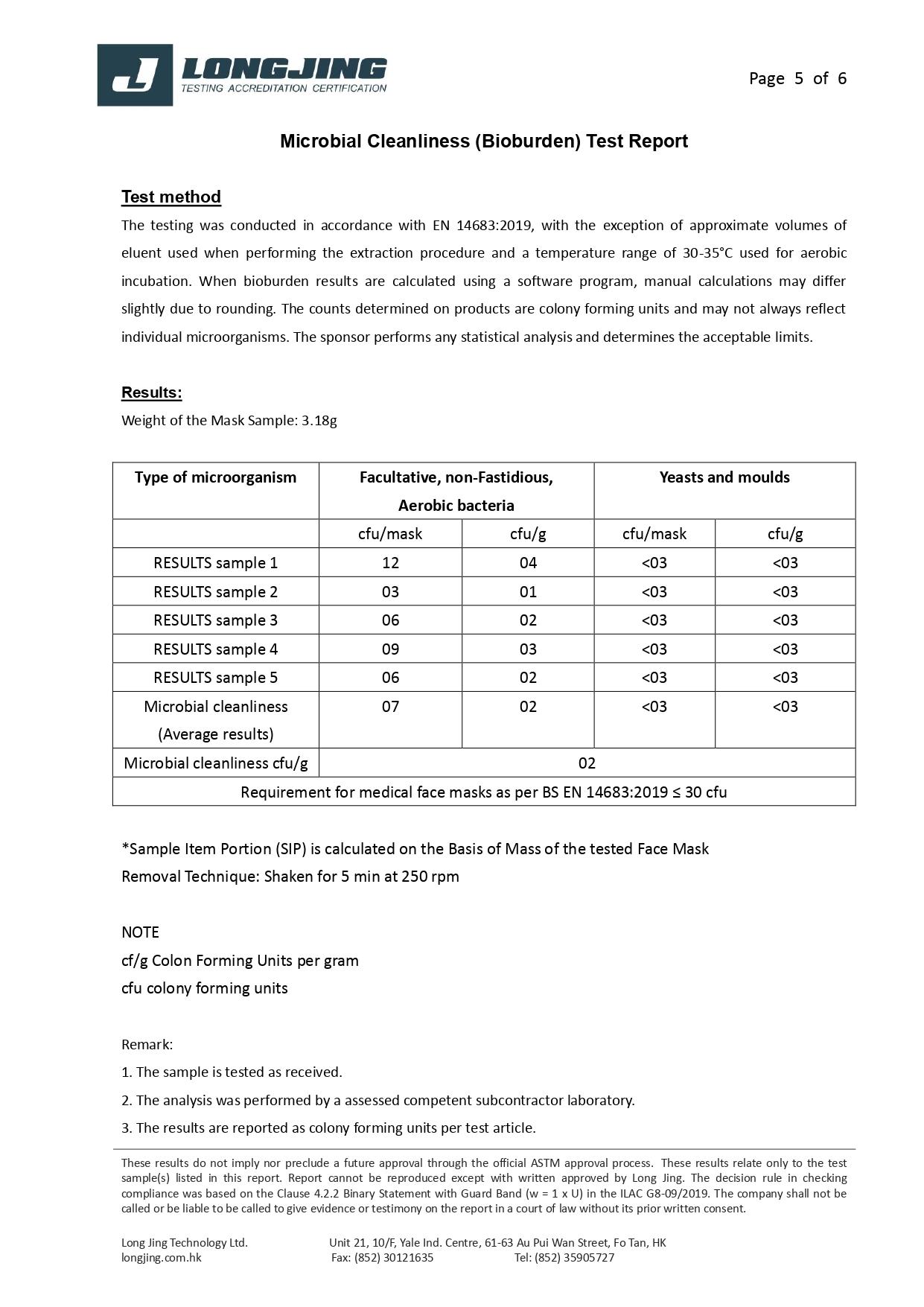
We are specialized in manufacturing 3-ply disposable medical face mask. Our factory is located at Hung Hom area (Hong Kong),and we have attained ISO 13485:2016 Medical Device Quality Management Systems Standard and ISO 14644-1:2015 Class 8 Cleanroom Qualification since 2020.
Certification of Product
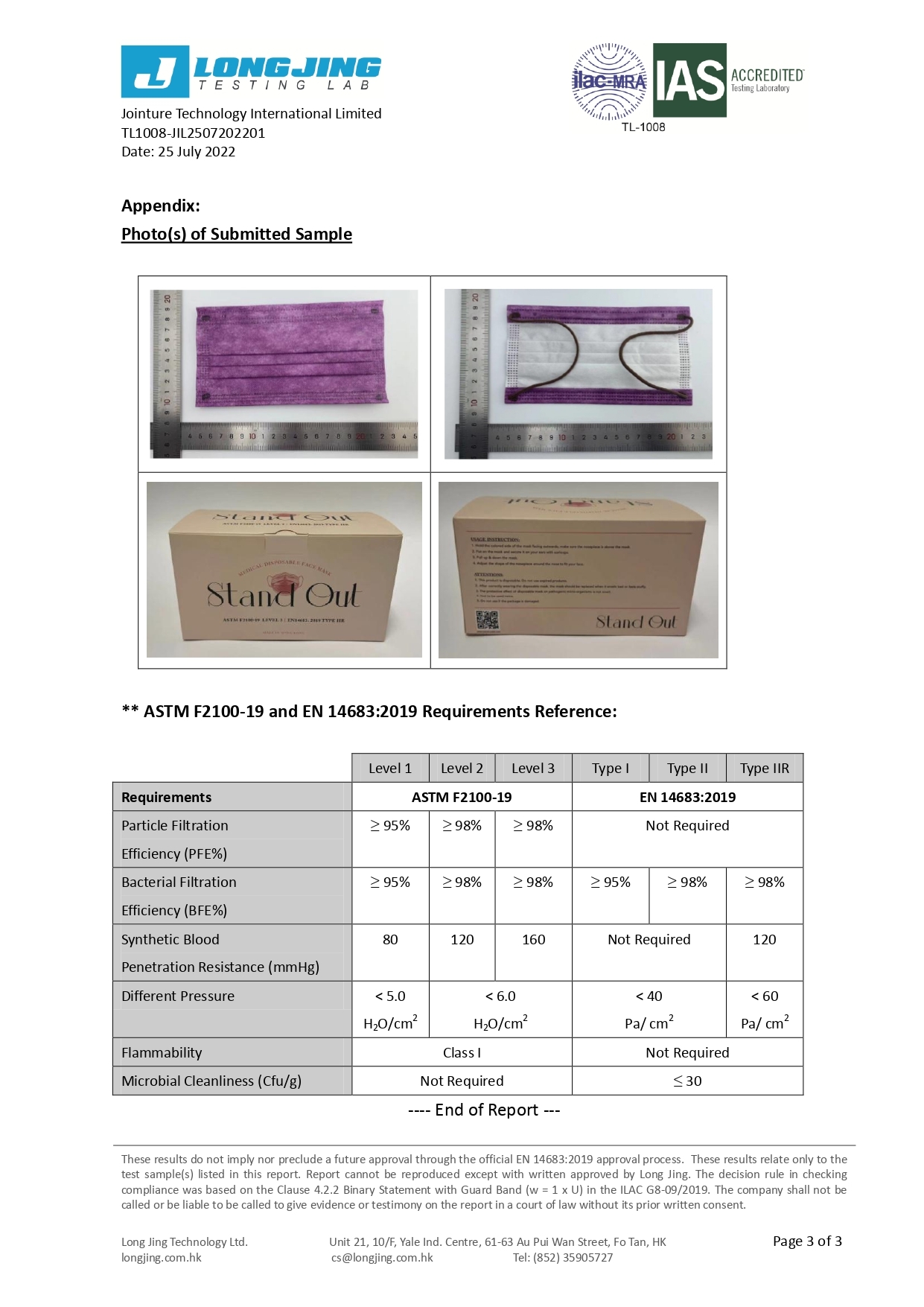
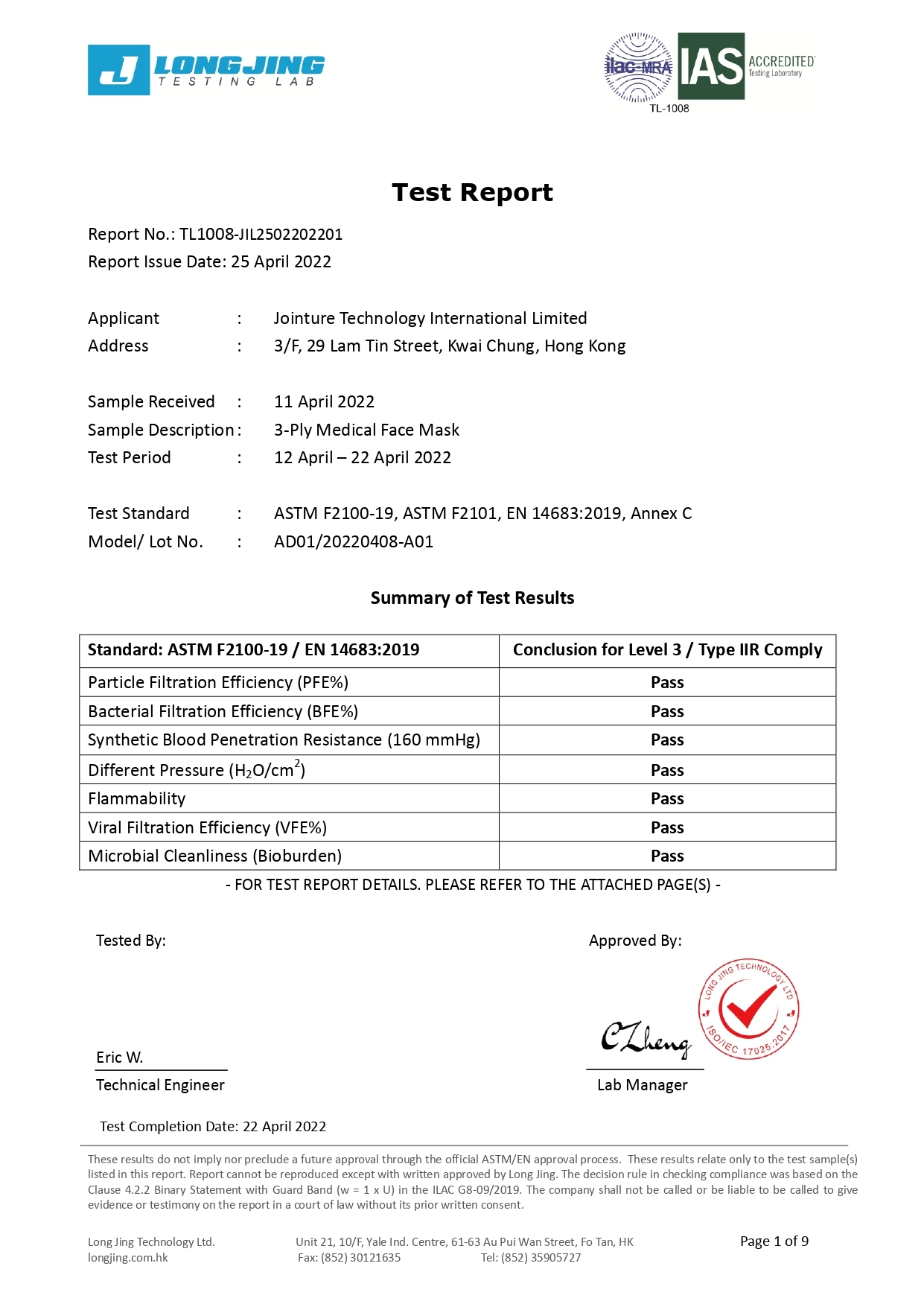
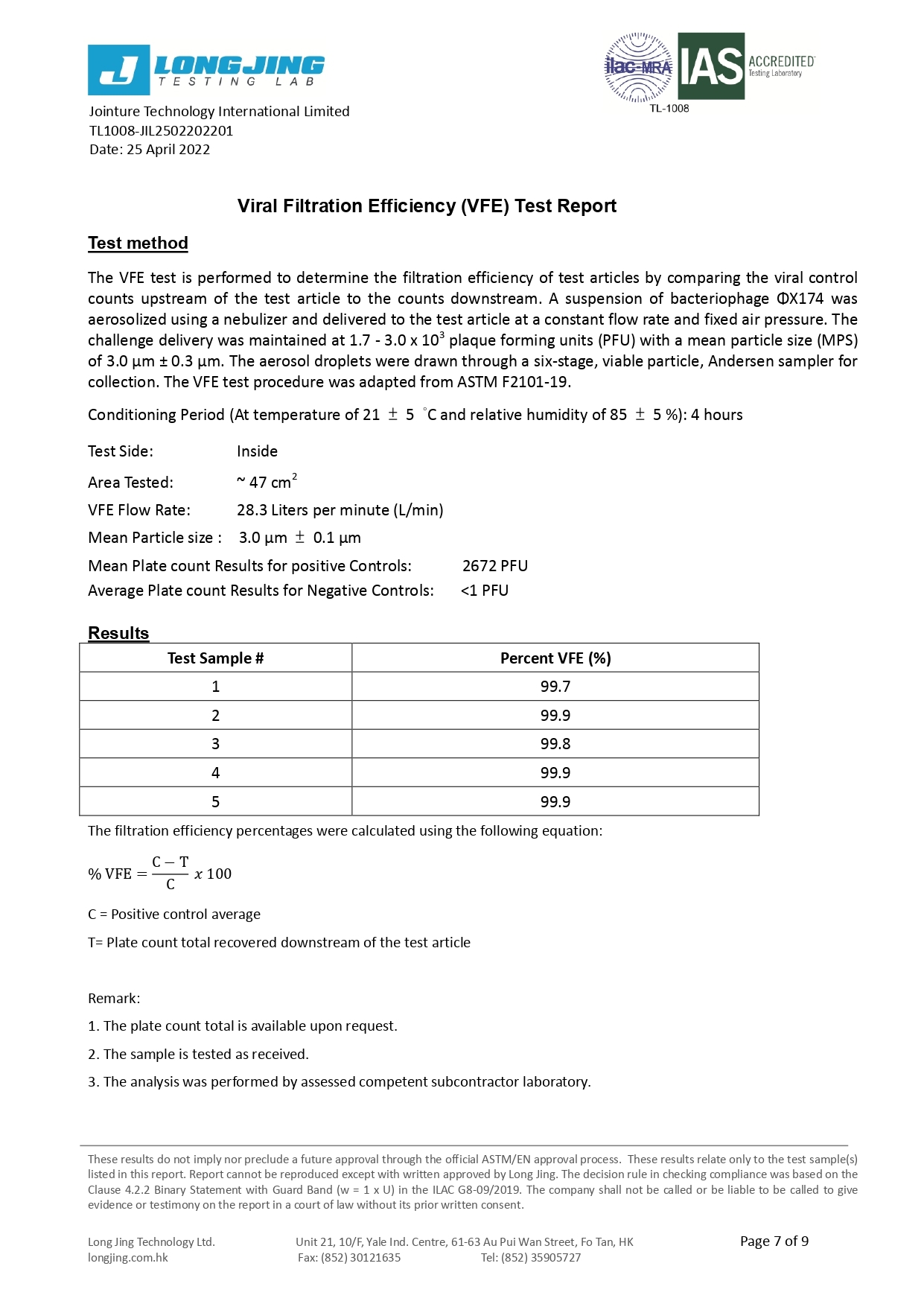
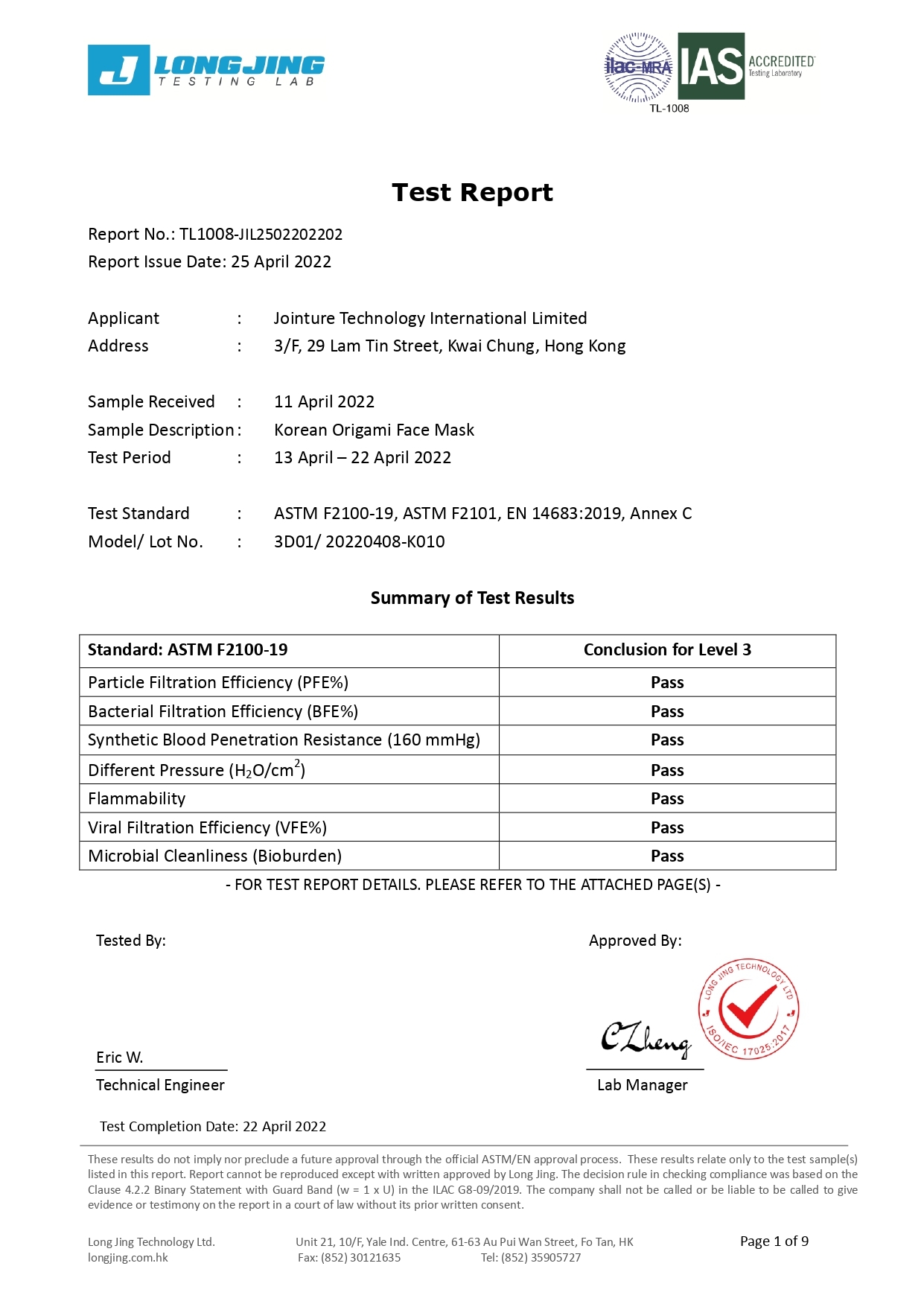
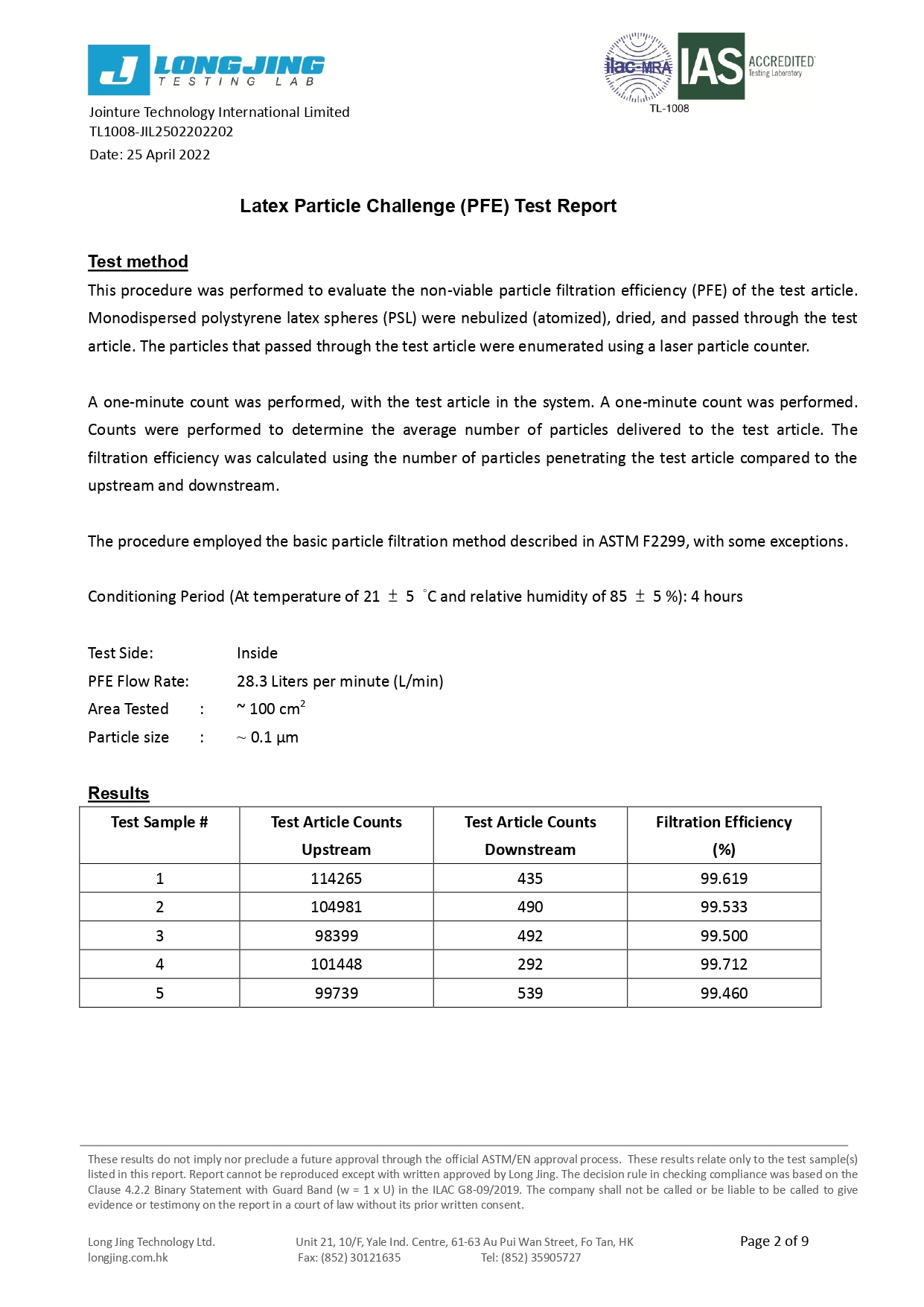
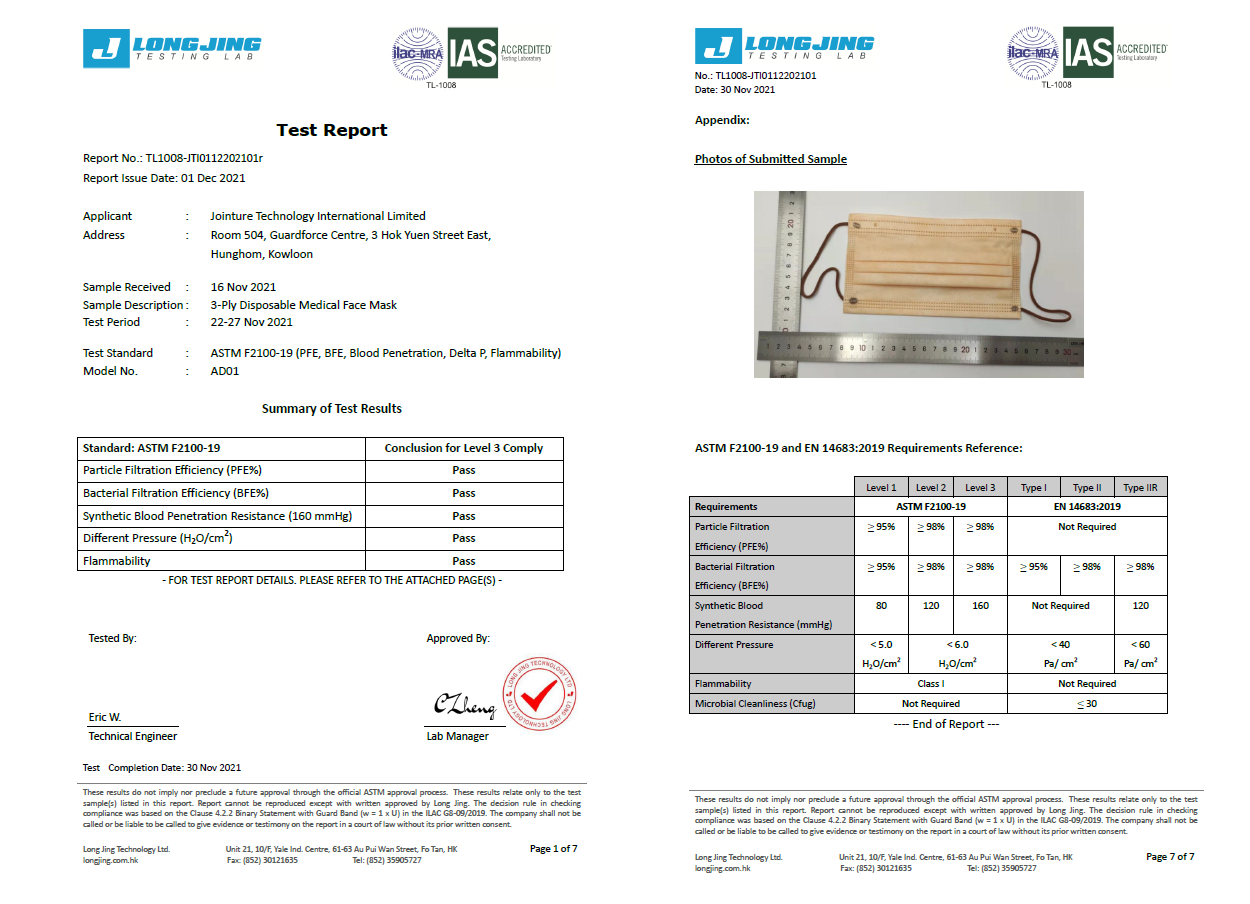
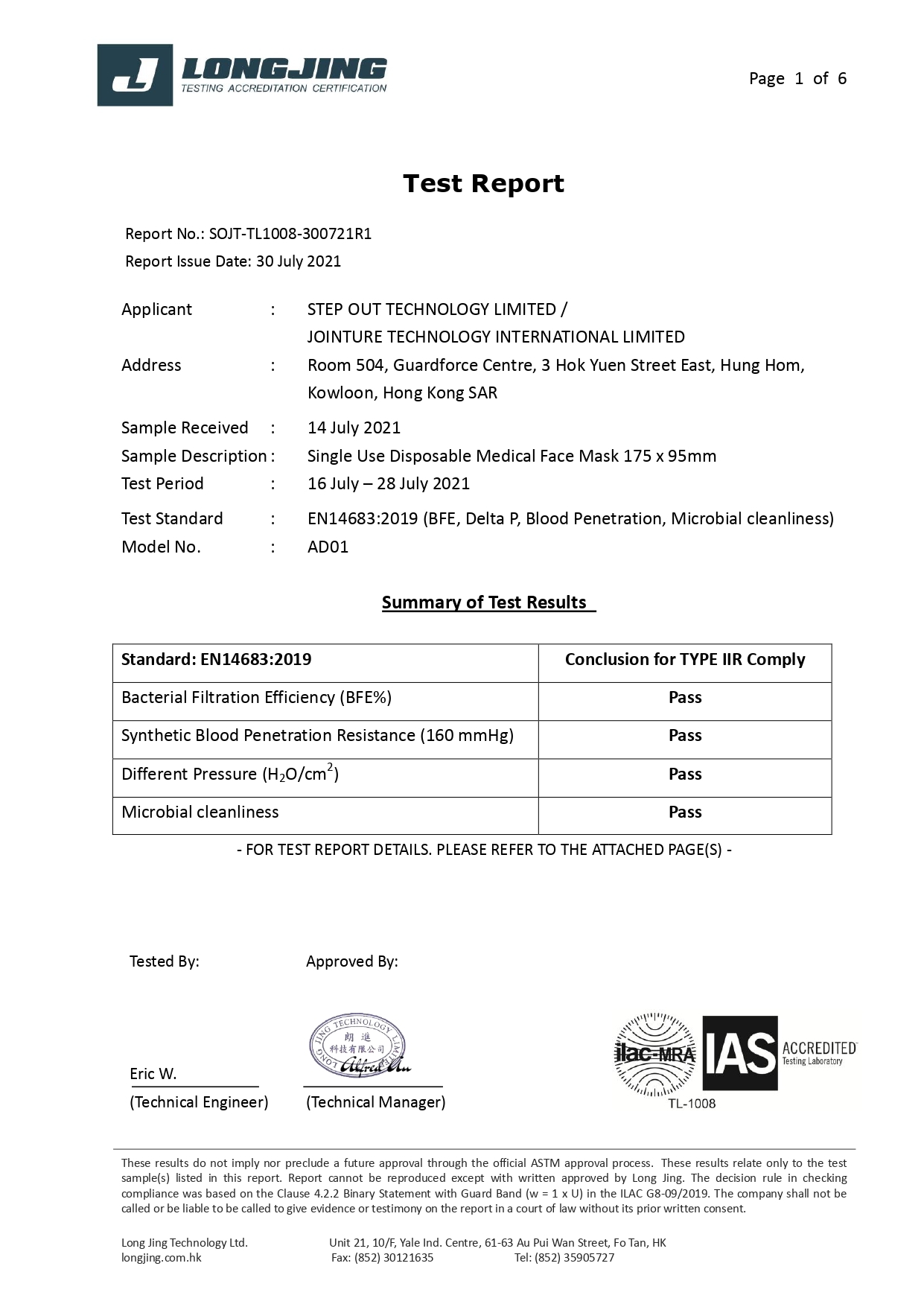
Our 3-ply disposal medical face masks are qualified for several international standard, including European Union EN14683:2019 Type IIR and U.S. ASTM F2100-19 Level 3.
Our products are also qualified for GB19083:2010 Physical Strength Test for Medical Protective Mask and GB15979-2002Hygienic Standard for Disposable Sanitary Products.
We are under the regulation of The Consumer Goods Safety Ordinance, Chapter 456, Laws of Hong Kong and the Trade Descriptions Ordinance, Chapter 362, Laws of Hong Kong, ensuring that our consumer goods supplied in Hong Kong Special Administrative Region (HKSAR) meet the general safety requirement, and prohibiting false trade descriptions, false, misleading or incomplete information and misstatements in respect of goods provided in the course of trade.
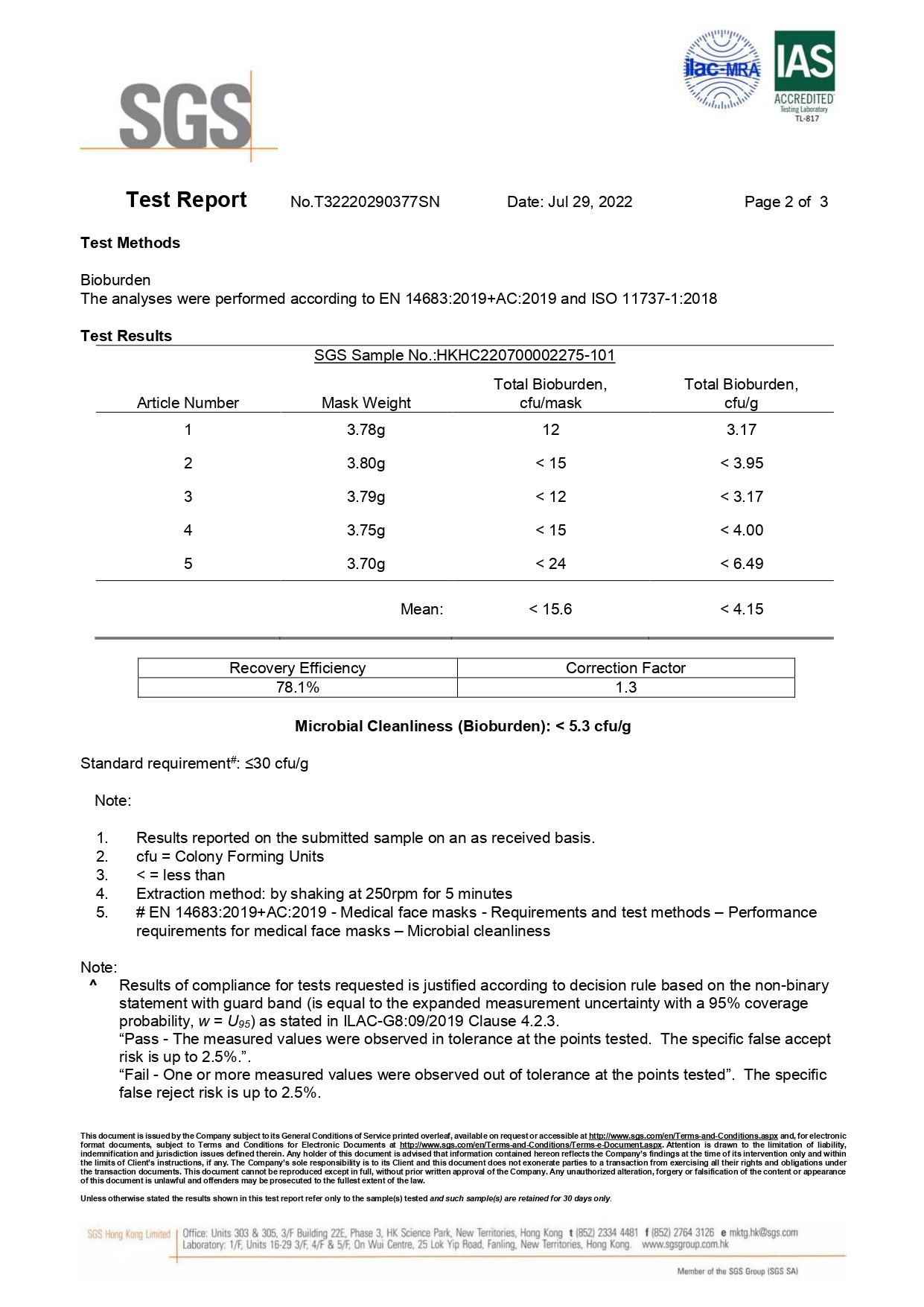
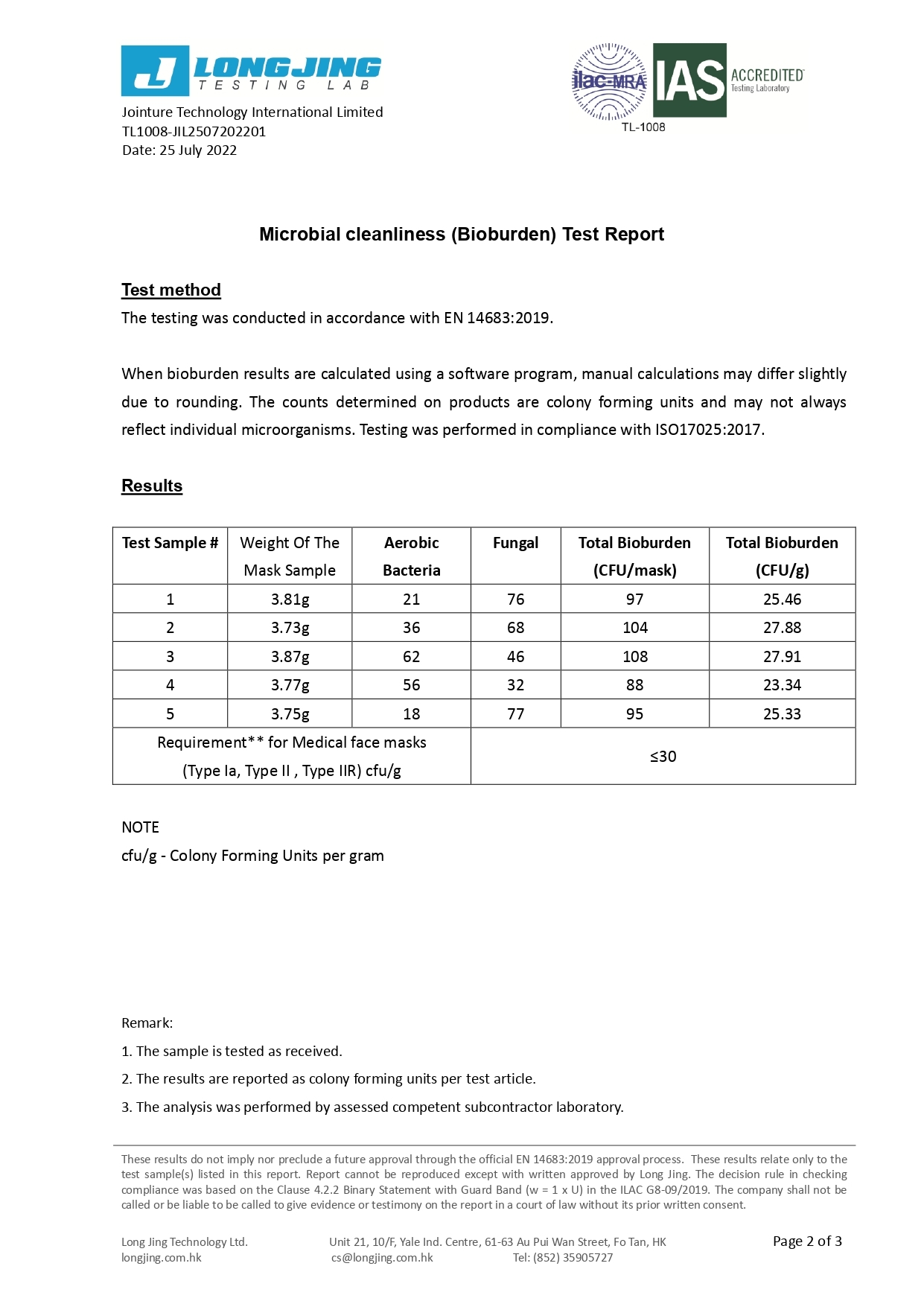
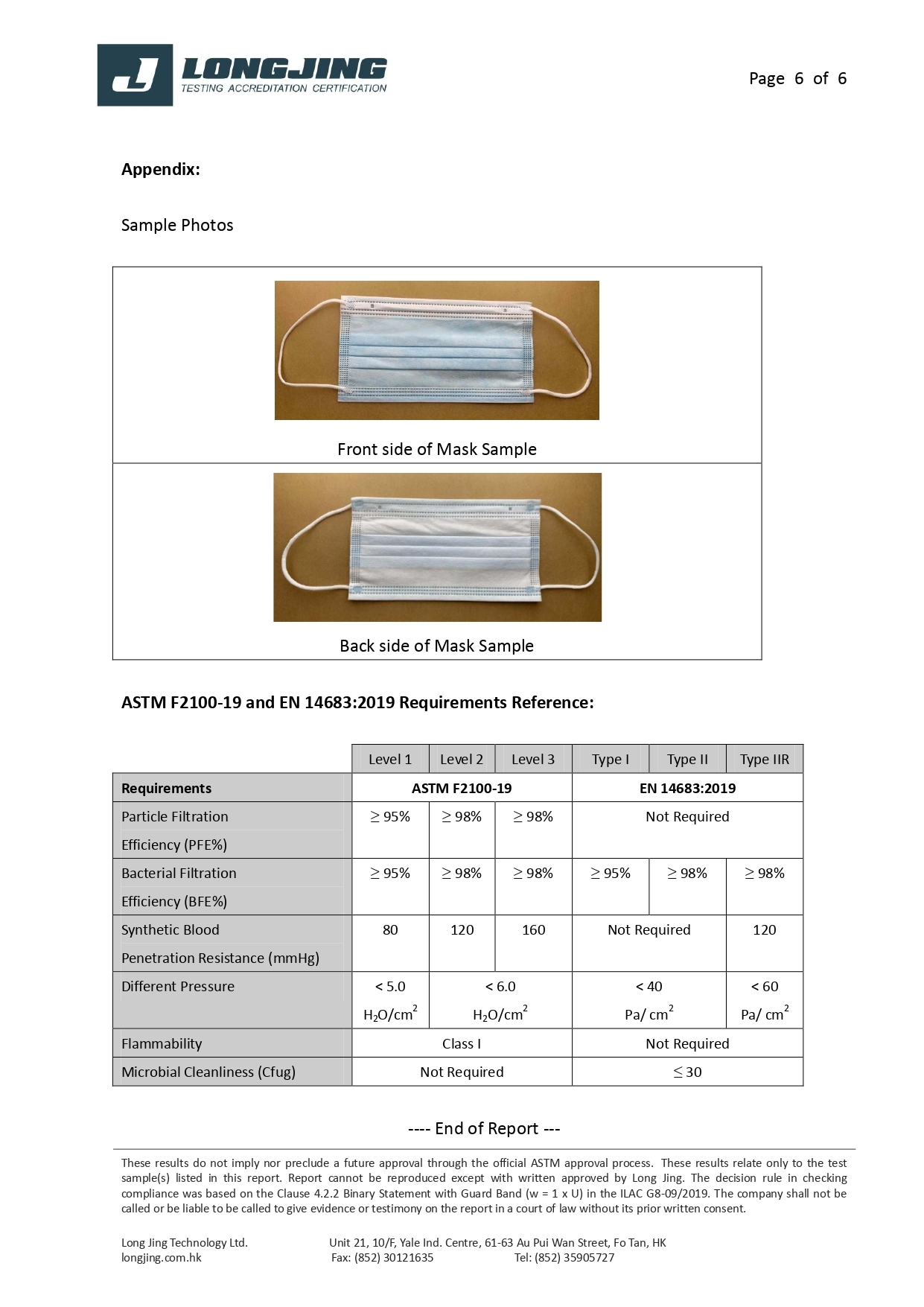
ASTM F2100-19 | Level 1 | Level 2 | Level 3 |
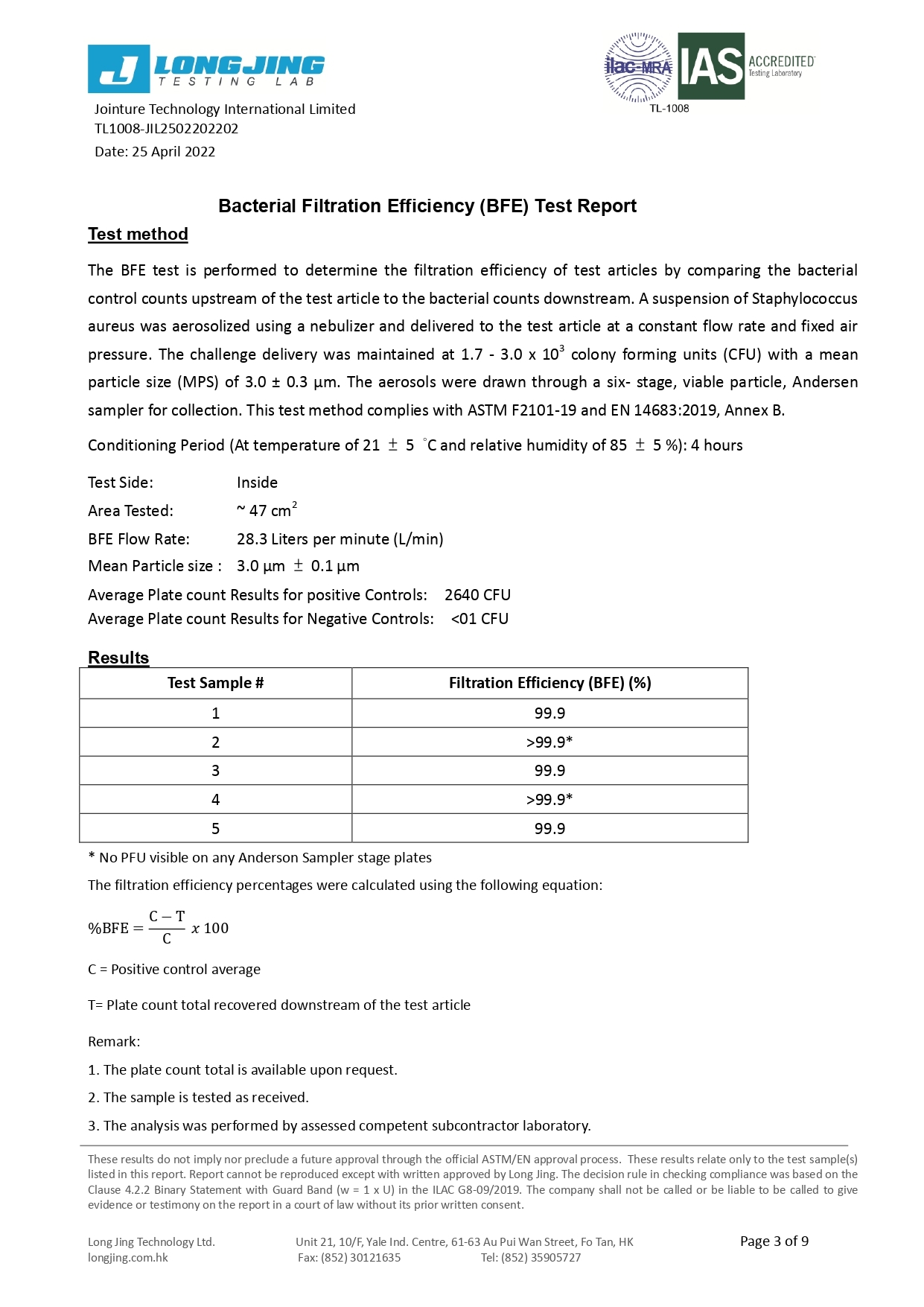
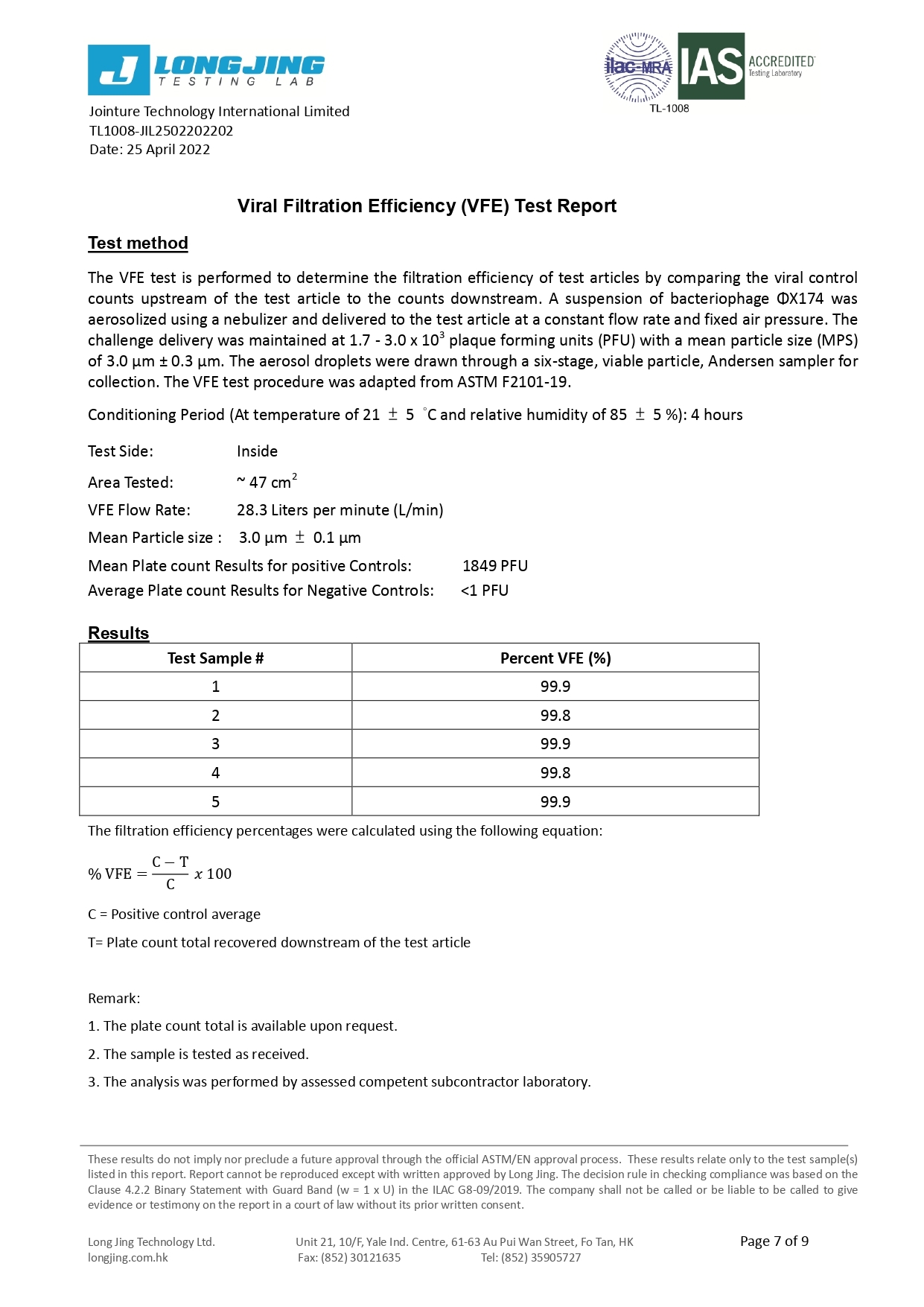
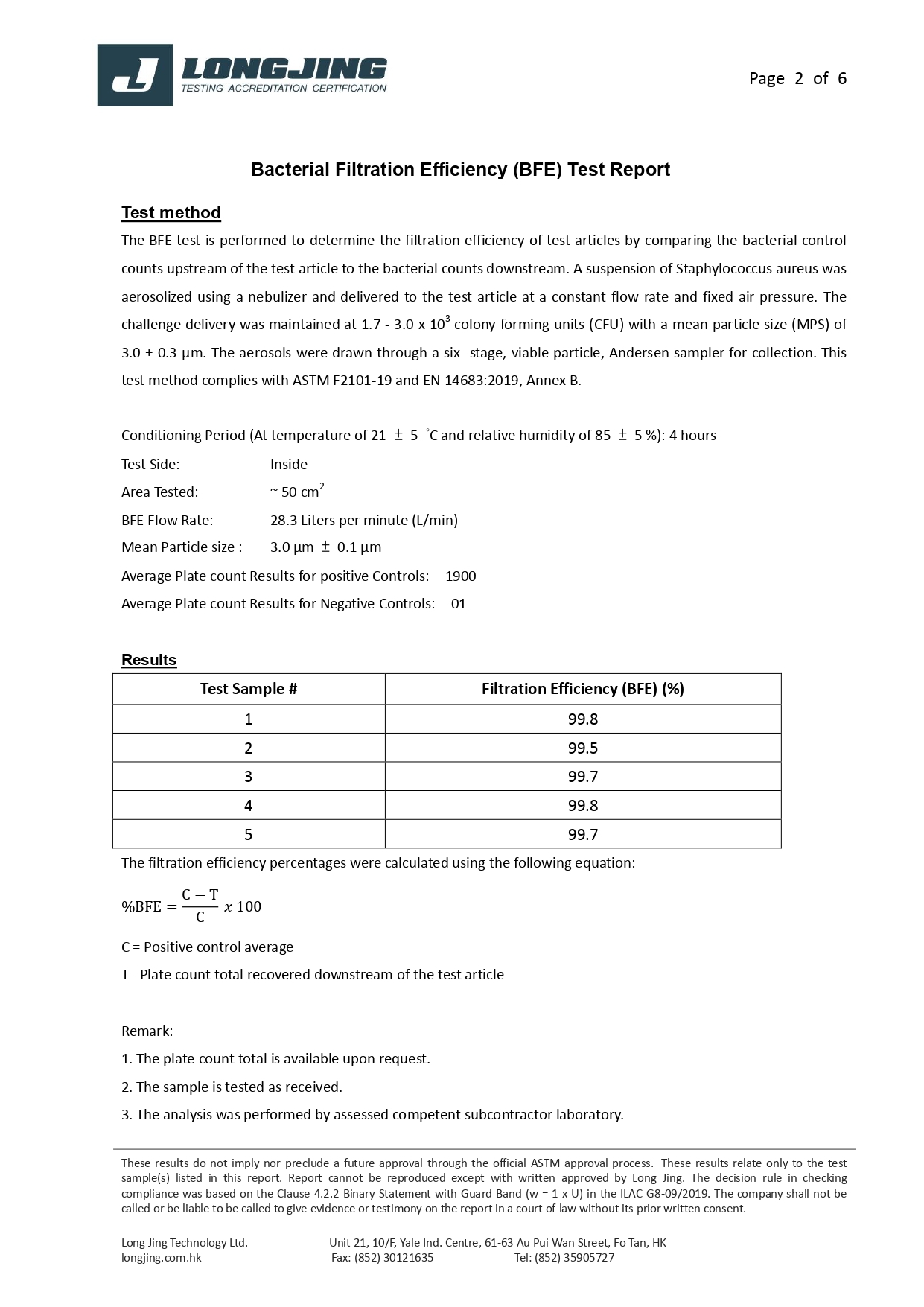
Bacterial Filtration Efficiency BFE % | ≥95 | ≥98 | ≥98 |
Particulate Filtration Efficiency PFE % @0.1mm | ≥95 | ≥98 | ≥98 |
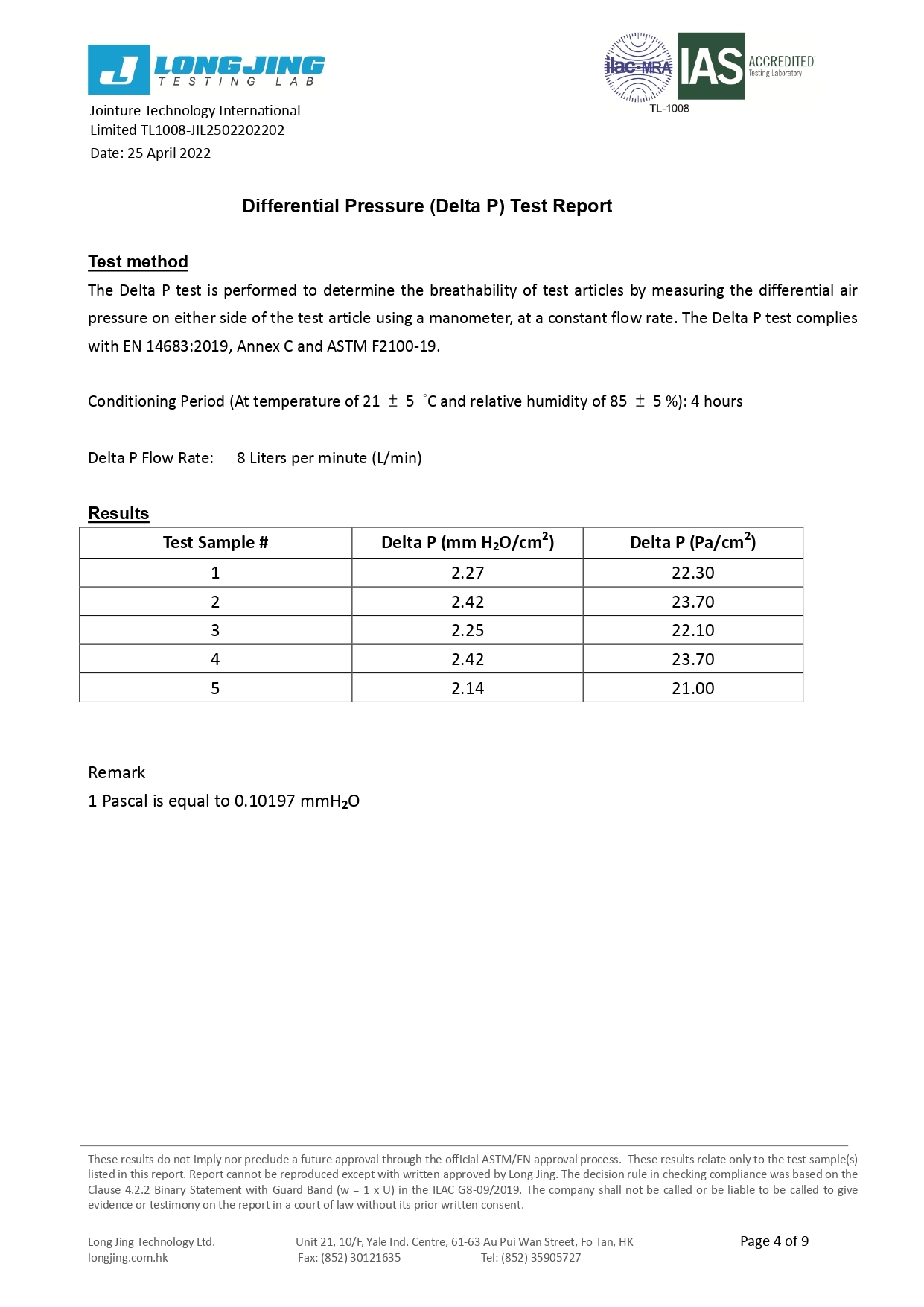
Differential pressure (Delta P) (mmH₂O/cm²) | <5.0 | <6.0 | <6.0 |
Synthetic Blood Penetration (mmHg) | 80 | 120 | 160 |
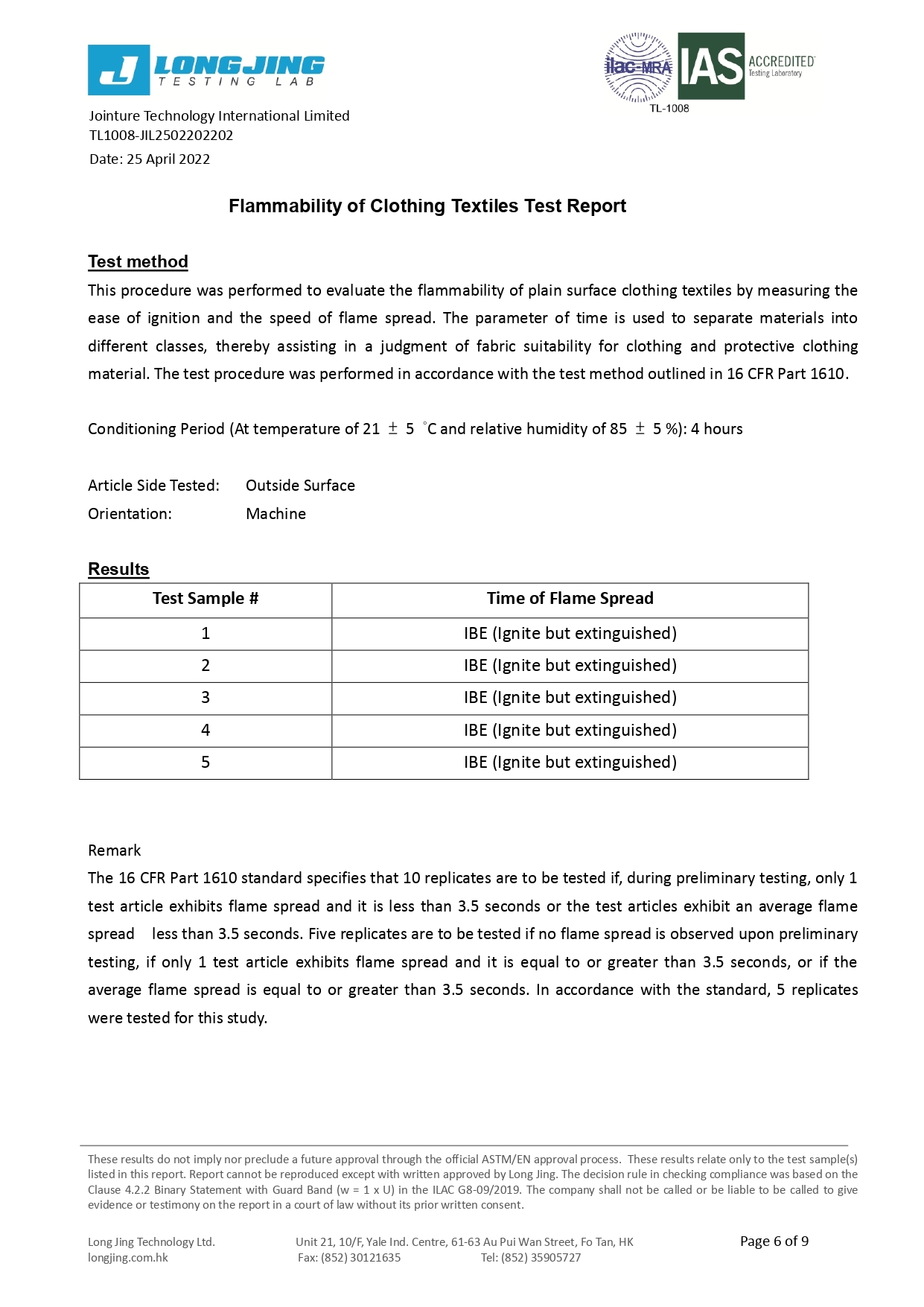
Flammability | Class 1 | Class 1 | Class 1 |
EN 14683: 2019 | Type I | Type II | Type IIR |
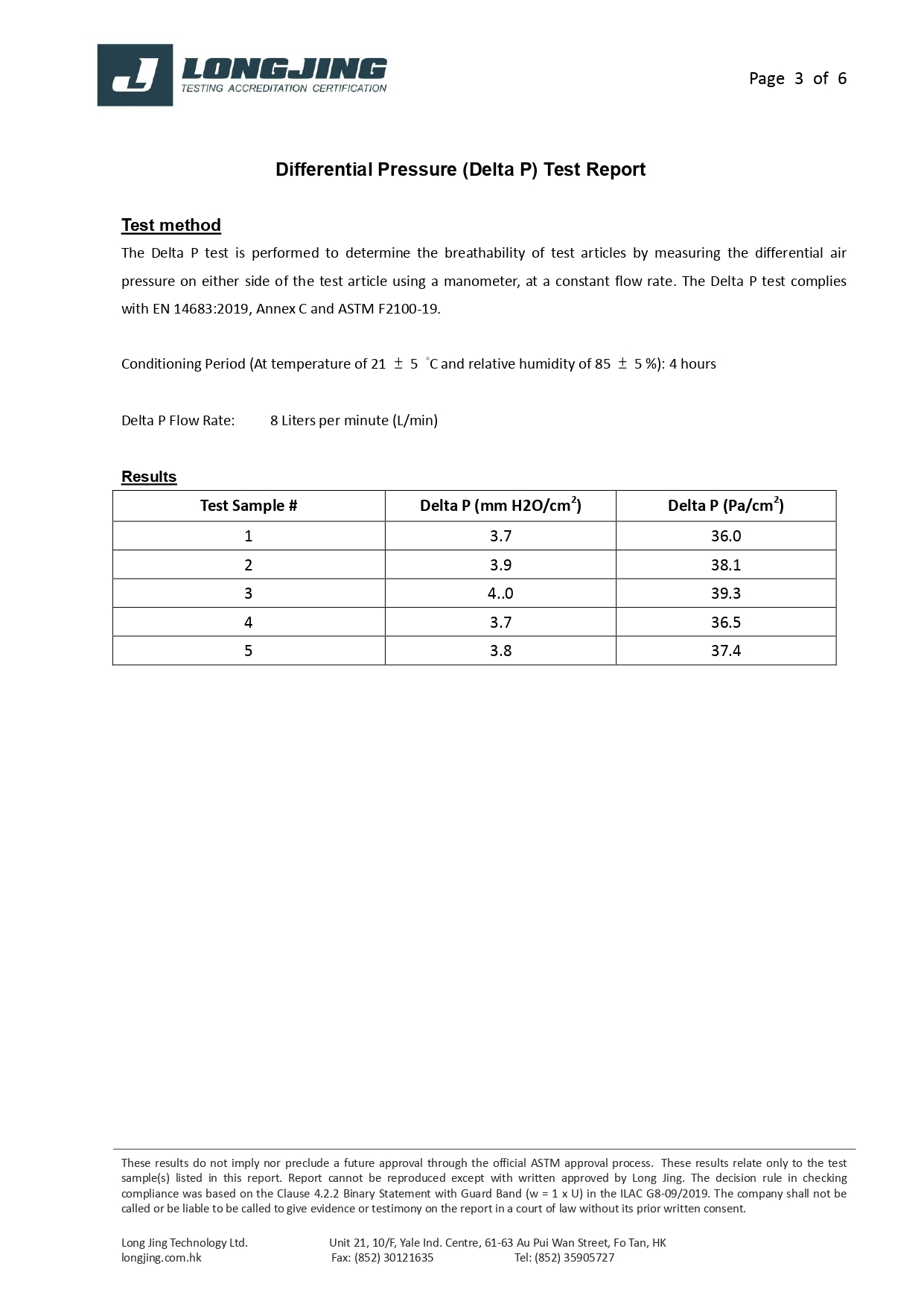
Bacterial Filtration Efficiency BFE % | ≥95 | ≥98 | ≥98 |
Differential pressure (Delta P) (Pa/cm²) | <40 | <40 | <60 |
Splash Resistance (kPa) | Not required | Not required | ≥16.0 (120 mmHg) |
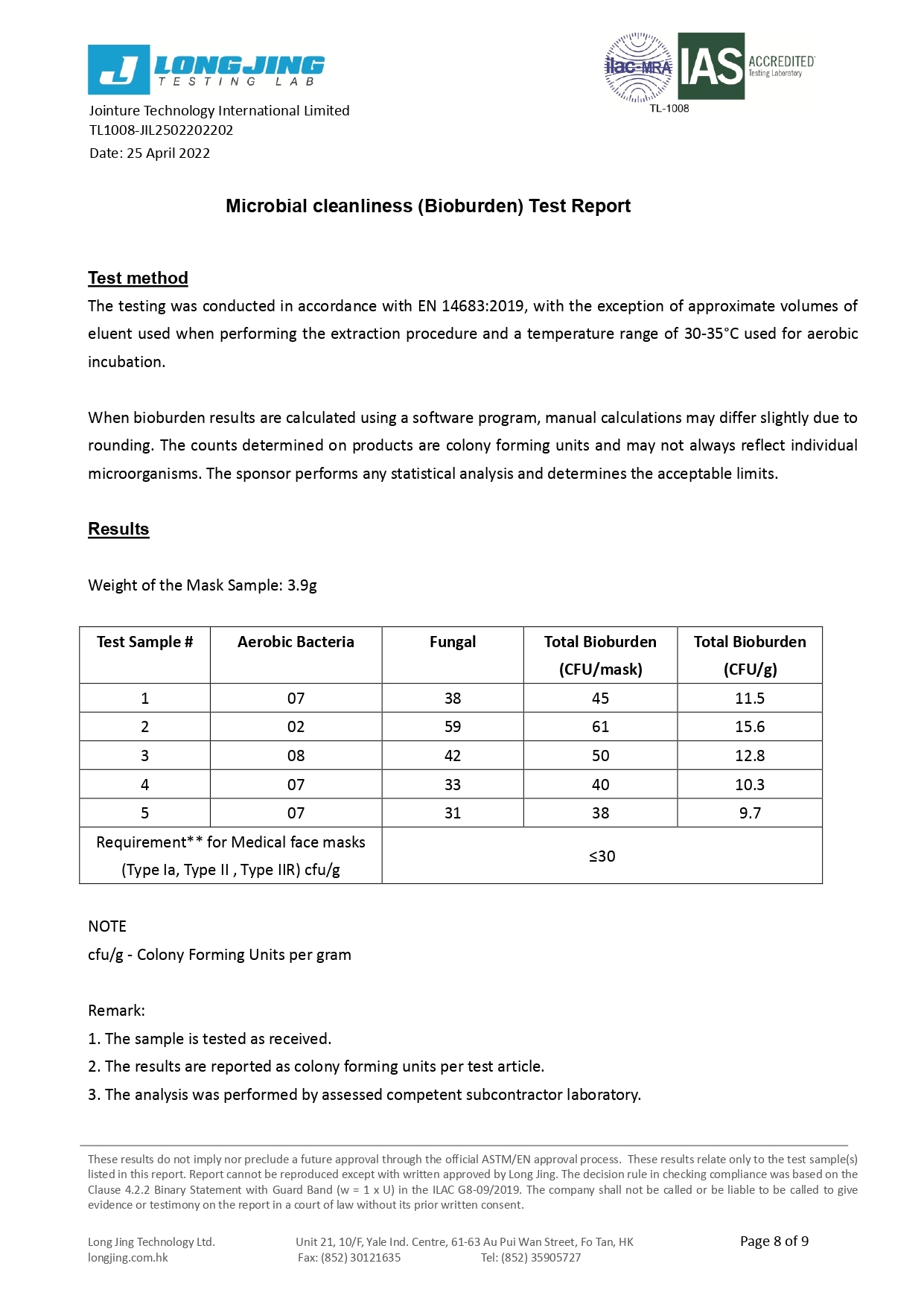
Microbial Cleanliness (CFU/g) | ≤30 | ≤30 | ≤30 |